Vaccine against hand-foot-mouth disease to be brought to Viet Nam
VGP - The Viet Nam Vaccine Joint Stock Company (VNVC) Immunization Center System and Swiss pharmaceutical firm Substipharm Biologics have signed a Memorandum of Understanding, opening opportunities to bring vaccine against hand-foot-mouth disease to Viet Nam.
The vaccine is currently awaiting approval by the Ministry of Health for distribution. Once approved, this would be Viet Nam's first HFMD vaccine, significantly improving disease prevention efforts in the Southeast Asian country.
The HFMD vaccine is recommended for children aged 2 months to under 6 years. It has been proven safe and provides up to 97 percent protection, offering long-term immunity against HFMD caused by the EV71 strain.
Clinical trials for the vaccine began in Chinese Taipei in 2010, with Phase 3 trials conducted in Viet Nam since 2019.
In Viet Nam, HFMD appears year-round, and peaks between March-April and September-December. The most recent outbreak in 2023 saw over 180,000 cases, 2.7 times higher than in 2022, with 31 fatalities.
In 2024 alone, more than 76,000 cases were reported nationwide.
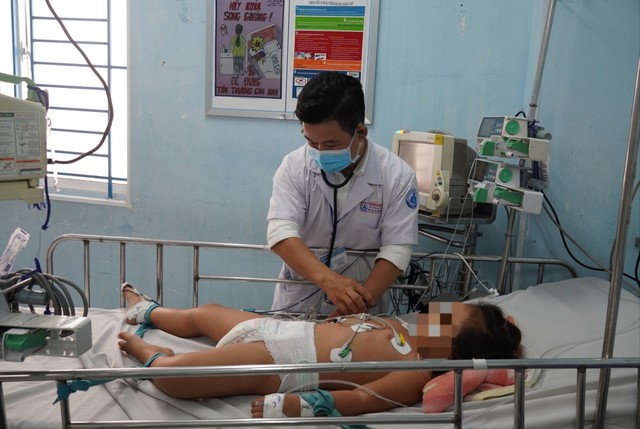
So far, hand, foot, and mouth disease still lacks a specific treatment without supportive care to help children through severe stages. The Pasteur Institute in Ho Chi Minh City has identified HFMD as a major public health challenge in Viet Nam.
The disease has three severity levels. Mild cases (Level 1) can be managed at home, while Levels 2-4 require hospitalization. Levels 2b, 3, and 4 may involve complications, necessitating close monitoring to prevent fatal outcomes.
In Viet Nam, the free National Expanded Program on Immunization (NEPI) was launched in 1981, providing immunization to 16 vaccine preventable diseases of Tuberculosis, Hepatitis B, Diphtheria, Pertussis, Tetanus, Polio, Hib, Measles, Rubella, Japanese Encephalitis, Cholera (in high-risk areas), and Typhoid (in high-risk areas), Rotavirus vaccine, Pneumococcal conjugate vaccine (PCV), Human papillomavirus (HPV) vaccine and seasonal influenza vaccine until 2030./.
